
You already saw that 1.00 g of al should yield 3.53 g of copper for the reaction below. 3cucl2(aq) + 2al(s)⟶ 2alcl3(aq) + 3cu(s) however, impurities and errors can occur easily. suppose that only 2.86 g of copper is produced. what is the percent yield of the reaction

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 16:00
Which process transfers heat from inside earth to its surface? convection currents in mantle pulling away of tectonic plates drawing in of tectonic plates convection currents in crust
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2

You know the right answer?
You already saw that 1.00 g of al should yield 3.53 g of copper for the reaction below. 3cucl2(aq) +...
Questions

Chemistry, 21.04.2021 17:00

Advanced Placement (AP), 21.04.2021 17:00



English, 21.04.2021 17:00


Arts, 21.04.2021 17:00




Mathematics, 21.04.2021 17:00

Mathematics, 21.04.2021 17:00


Mathematics, 21.04.2021 17:00

Mathematics, 21.04.2021 17:00

English, 21.04.2021 17:00




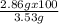 = 81 %
= 81 %

