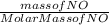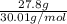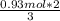Chemistry, 31.08.2019 18:10 jesh0975556
In the following reaction, how many grams of ammonia (nh3) will react with 27.8 grams of nitric oxide (no)? 4nh3 + 6no → 5n2 + 6h2o the molar mass of ammonia is 17.0337 grams and that of nitric oxide is 30.01 grams.
a- 23.7 grams
b- 10.5 grams
c- 73.5 grams
d- 32.7 grams

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2


Chemistry, 22.06.2019 17:30
The polymer used for the nonstick surface of cooking utensils is 24.0%c and 76%f by mass. what is the empirical formula of this polymer?
Answers: 2
You know the right answer?
In the following reaction, how many grams of ammonia (nh3) will react with 27.8 grams of nitric oxid...
Questions


Biology, 07.10.2019 19:30

Social Studies, 07.10.2019 19:30

Social Studies, 07.10.2019 19:30

Business, 07.10.2019 19:30

Mathematics, 07.10.2019 19:30


Biology, 07.10.2019 19:30


Social Studies, 07.10.2019 19:30



Chemistry, 07.10.2019 19:30

Mathematics, 07.10.2019 19:30

Chemistry, 07.10.2019 19:30


Mathematics, 07.10.2019 19:30



Mathematics, 07.10.2019 19:30