The following balanced equation shows the formation of water.
2h2 + o2 → 2h2o
how many...

Chemistry, 21.12.2019 00:31 mgrvashvi6698
The following balanced equation shows the formation of water.
2h2 + o2 → 2h2o
how many moles of oxygen (o2) are required to react completely with 1.67 mol h2?
a) 0.835 mol o2
b) 1.67 mol o2
c) 3.34 mol o2

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1

Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3


Chemistry, 23.06.2019 04:20
The equation below shows the reaction of zinc with hydrochloric acid (hcl). zn (s) + 2 hcl (aq) —> zncl2 (aq) + h2 (g) what will happen if the concentration of hcl is decreased? a. more zncl2 will be produced. b. the reaction rate will slow down. c. the hydrochloric acid will become more acidic. d. the reaction will produce water instead of hydrogen gas.
Answers: 1
You know the right answer?
Questions

Mathematics, 10.07.2019 05:30




Mathematics, 10.07.2019 05:30

Biology, 10.07.2019 05:30


Mathematics, 10.07.2019 05:30





Biology, 10.07.2019 05:30





History, 10.07.2019 05:30


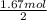
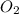
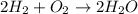
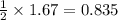 moles of oxygen.
moles of oxygen.

