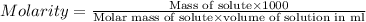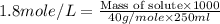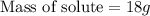Suppose you wanted to make an aqueous solution of sodium hydroxide that is 1.8 m. you want to make 250 ml of the solution. how many grams of sodium hydroxide are needed to make this solution? note which type of calculation you need to perform, and then perform the calculation.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 05:00
You mix the pks of succinic acid are 4.21 and 5.64. how many gramsa graduate student at sdsu wants to measure the activity of a particular enzyme at ph 4.0. to buffer her reaction, she will use a buffer system based on one of the acids listed below, which acid is most appropriate for the experiment? of monosodium succinate (fw = 140 g/mol) and disodium succinate (fw = 162 g/mol) must be added to 1 l of water to produce a solution with a ph 5.28 and a total solute concentration of 100 mm? (assume the total volume remains 1 liter, answer in grams monosodium succinate, grams disodium succinate, respectively.) volumes of 0.05 m nah2po4 and 0.05 m na2hpo4 (pk's for phosphoric acid are 2.15, 6.82 and 12.38). which of the following best describes the resulting solution?
Answers: 2

Chemistry, 22.06.2019 14:00
Anthracite is so hard and pure it is also referred to as a renewable resource metamorphic rock hot bituminous coal dirty fuel
Answers: 1

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1
You know the right answer?
Suppose you wanted to make an aqueous solution of sodium hydroxide that is 1.8 m. you want to make 2...
Questions

English, 26.01.2021 08:00


Mathematics, 26.01.2021 08:00


English, 26.01.2021 08:00


Mathematics, 26.01.2021 08:00

Mathematics, 26.01.2021 08:00


Mathematics, 26.01.2021 08:00


Mathematics, 26.01.2021 08:00

History, 26.01.2021 08:00




Mathematics, 26.01.2021 08:00

Chemistry, 26.01.2021 08:00

Mathematics, 26.01.2021 08:00