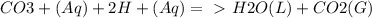Chemistry, 21.09.2019 08:50 yarrito20011307
Every antacid contains one or more ingredients capable of reacting with excess stomach acid (hcl). the essential neutralization products are co2 and/or h2o. write net ionic equations to represent the neutralizing action of the following popular antacids:
-rolaids
-maalox
-tums
-milk of magnesia

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1



Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
Every antacid contains one or more ingredients capable of reacting with excess stomach acid (hcl). t...
Questions


History, 29.01.2020 16:53




Spanish, 29.01.2020 16:53


Mathematics, 29.01.2020 16:53

English, 29.01.2020 16:53


Business, 29.01.2020 16:53


Mathematics, 29.01.2020 16:53



Mathematics, 29.01.2020 16:53

Mathematics, 29.01.2020 16:53


Physics, 29.01.2020 16:53

Mathematics, 29.01.2020 16:53