

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2


Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
The fuel used in many disposable lighters is liquid butane, c4h10. butane has a molecular weight of...
Questions


History, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00


Mathematics, 30.04.2021 14:00



Mathematics, 30.04.2021 14:00



English, 30.04.2021 14:00

English, 30.04.2021 14:00


Social Studies, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00

English, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00

Mathematics, 30.04.2021 14:00


Health, 30.04.2021 14:00


 = 58.1 g/mole
= 58.1 g/mole
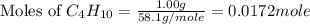
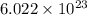 number of atoms.
number of atoms.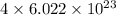 number of carbon atoms.
number of carbon atoms. number of carbon atoms.
number of carbon atoms.

