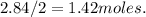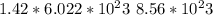13. solid sodium reacts violently with water producing heat, hydrogen gas and sodium hydroxide. how many molecules of hydrogen gas are produced when 65.4 g of sodium are added to water? 2na(s) + 2h2o(l) → 2naoh (aq) + h2(g)(1 point for molar mass of sodium, 1 point for correct mole ratio, 1 point for work, 1 point for correct answer with correct units) (4 points total) *
your answer

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

You know the right answer?
13. solid sodium reacts violently with water producing heat, hydrogen gas and sodium hydroxide. how...
Questions






Mathematics, 03.03.2020 18:23

Mathematics, 03.03.2020 18:23








Mathematics, 03.03.2020 18:23




Biology, 03.03.2020 18:23