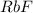Chemistry, 29.01.2020 22:53 brittanyelliott028
Asalt is known to be an alkali metal fluoride. a quick approximate determination of freezing point indicates that 4 g of the salt dissolved in 100 g of water produces a solution that freezes at about −1.4 °c. what is the formula of the salt? show your calculations.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Why is an elements atomic mass not listed as a whole number on the periodic table
Answers: 2

Chemistry, 21.06.2019 16:00
If the same amount of cacl2 is added to equal volumes of water and maple syrup, which will have the higher temperature?
Answers: 1


Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1
You know the right answer?
Asalt is known to be an alkali metal fluoride. a quick approximate determination of freezing point i...
Questions


Health, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00


English, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00



History, 26.02.2021 21:00



Mathematics, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00


Mathematics, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00

Mathematics, 26.02.2021 21:00

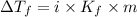
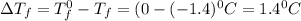 = Depression in freezing point
= Depression in freezing point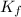 = freezing point constant =
= freezing point constant = 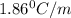
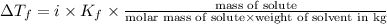
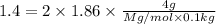
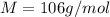
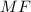 is 106 g/mol
is 106 g/mol