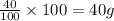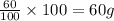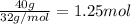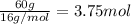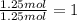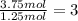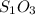Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 15:00
According to the diagram, what sources contribute to the phosphorus found in soil? according to the diagram, phosphorus found in soil contributes phosphorus to what other sources?
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
What is the empirical formula of a compound that is 40% sulfur and 60% oxygen by weight?...
Questions


Law, 20.11.2020 05:10

World Languages, 20.11.2020 05:10

Business, 20.11.2020 05:10

Mathematics, 20.11.2020 05:10

Mathematics, 20.11.2020 05:10

Law, 20.11.2020 05:10

Mathematics, 20.11.2020 05:10


Mathematics, 20.11.2020 05:10


English, 20.11.2020 05:10

History, 20.11.2020 05:10

Mathematics, 20.11.2020 05:10

Chemistry, 20.11.2020 05:10


English, 20.11.2020 05:10

Mathematics, 20.11.2020 05:10


Social Studies, 20.11.2020 05:10

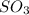 .
.