
Chemistry, 14.01.2020 05:31 makaileep7449
What are the steps needed when solving problem like this?
a compound of uranium and fluorine is used to generate uranium for nuclear power plants. the gas can be decomposed to yield 2.09 parts by mass of uranium for every 1 part by mass of fluorine. if the relative mass of a uranium atom is 238 and the relative mass of a fluorine atom is 19, calculate the number of fluorine atoms that are combined with one uranium atom.
search entries or author
filter replies by unread

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:10
When will le chatelier's principle come into effect? at the beginning of a reaction, when there are only reactants when a reaction has reached chemical equilibrium when a catalyst is added to a reaction mixture when a reaction is occurring but not yet at equilibrium
Answers: 3


Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
What are the steps needed when solving problem like this?
a compound of uranium and fluorine...
a compound of uranium and fluorine...
Questions

History, 11.03.2021 18:40


History, 11.03.2021 18:40



Mathematics, 11.03.2021 18:40


Physics, 11.03.2021 18:40

Chemistry, 11.03.2021 18:40

Mathematics, 11.03.2021 18:40

English, 11.03.2021 18:40

History, 11.03.2021 18:40

Mathematics, 11.03.2021 18:40


Spanish, 11.03.2021 18:40


Social Studies, 11.03.2021 18:40



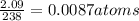
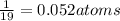
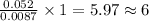 atoms of Fluorine atoms.
atoms of Fluorine atoms.

