Which statement best describes a bronsted-lowry base?
a) it must accept protons to form a con...

Chemistry, 31.01.2020 23:01 jaymoney0531
Which statement best describes a bronsted-lowry base?
a) it must accept protons to form a conjugate base.
b) it must accept protons to form a conjugate acid.
c) it must donate protons to form a conjugate base.
d) it must donate protons to form a conjugate acid.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1


Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 22.06.2019 22:40
Percent ionization for a weak acid (ha) is determined by the following formula: percent ionization=[ha] ionized[ha] initial×100%for strong acids, ionization is nearly complete (100%) at most concentrations. however, for weak acids, the percent ionization changes significantly with concentration. the more diluted the acid is, the greater percent ionization.a certain weak acid, ha, has a ka value of 9.4×10? 7.part acalculate the percent ionization of ha in a 0.10 m solution.part bcalculate the percent ionization of ha in a 0.010 m solution
Answers: 1
You know the right answer?
Questions

Spanish, 04.12.2019 15:31


Mathematics, 04.12.2019 15:31

Mathematics, 04.12.2019 15:31

Arts, 04.12.2019 15:31

History, 04.12.2019 15:31

Biology, 04.12.2019 15:31

Mathematics, 04.12.2019 15:31

Mathematics, 04.12.2019 15:31

Social Studies, 04.12.2019 15:31

Mathematics, 04.12.2019 15:31

Mathematics, 04.12.2019 15:31

Chemistry, 04.12.2019 15:31

Social Studies, 04.12.2019 15:31





Chemistry, 04.12.2019 15:31

Mathematics, 04.12.2019 15:31

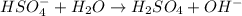
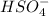 is a Bronsted-Lowry base because it can accept proton or hydrogen ion from
is a Bronsted-Lowry base because it can accept proton or hydrogen ion from 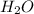 .
. 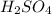 as conjugate acid.
as conjugate acid.

