
Chemistry, 29.01.2020 00:52 waterdrop026
The reaction ab(aq)→a(g)+b(g) is second order in ab and has a rate constant of 0.0164 m −1 ⋅ s −1 at 25.0 ∘ c . a reaction vessel initially contains 250.0 ml of 0.104 m ab which is allowed to react to form the gaseous product. the product is collected over water at 25.0 ∘ c . part a how much time is required to produce 142.0 ml of the products at a barometric pressure of 707.3 mmhg . (the vapor pressure of water at this temperature is 23.8 mmhg .)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2


Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 18:10
Areader can tell that the meaning of “obnoxious” will include “having the quality of something” because of the .a) prefix b)pronunciation c)suffix d) word root
Answers: 3
You know the right answer?
The reaction ab(aq)→a(g)+b(g) is second order in ab and has a rate constant of 0.0164 m −1 ⋅ s −1 at...
Questions


German, 25.05.2021 20:50



Mathematics, 25.05.2021 20:50

Mathematics, 25.05.2021 20:50

Mathematics, 25.05.2021 20:50


Biology, 25.05.2021 20:50




Biology, 25.05.2021 20:50

Biology, 25.05.2021 20:50

Mathematics, 25.05.2021 20:50

Mathematics, 25.05.2021 20:50


English, 25.05.2021 20:50


![v=k[AB]^2](/tpl/images/0479/4220/bde1b.png) . In the statement, we obtain that
. In the statement, we obtain that ![[AB]=0.104~M](/tpl/images/0479/4220/4f567.png) and, at 25 ºC,
and, at 25 ºC, 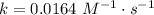 . Then:
. Then:![v=k[AB]^2\\\\ v=0.0164\cdot0.104^2\\\\ v=0.0164\cdot0.010816\\\\ v\approx0.000177=1.77\times10^{-4}~mol/s](/tpl/images/0479/4220/68b4c.png)
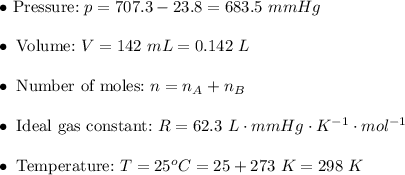

 . Hence:
. Hence: 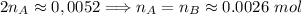 .
.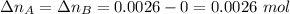 . Furthermore, since the ratio of AB to A and to B is 1:1,
. Furthermore, since the ratio of AB to A and to B is 1:1, 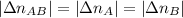 .
.![v=\dfrac{|\Delta[AB]|}{\Delta t}=\dfrac{1}{\Delta t}\cdot\dfrac{|\Delta n_{AB}|}{V}=\dfrac{1}{\Delta t}\cdot\dfrac{|\Delta n_A||}{250~mL}\\\\ 1.77\cdot10^{-4}=\dfrac{1}{\Delta t}\cdot\dfrac{0.0026~mol}{0.25~L}\\\\ \Delta t=\dfrac{0.0026}{0.25\cdot1.77\cdot10^{-4}}\\\\ \boxed{\Delta t\approx58.76~s}](/tpl/images/0479/4220/8e656.png)


