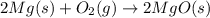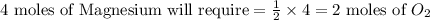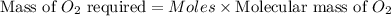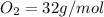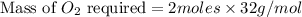Chemistry, 23.01.2020 14:31 blessed4628
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium oxide (mgo).
2mg + o2 mc009-1.jpg 2mgo
the molar mass of o2 is 32.0 g/mol. what mass, in grams, of o2 is required to react completely with 4.00 mol of mg?
2.00

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

Chemistry, 23.06.2019 13:50
Use the periodic table and your knowledge of isotopes to complete these statements. when polonium-210 emits an alpha particle, the child isotope has an atomic mass of 1-131 undergoes beta-minus decay. the chemical symbol for the new element is fluorine-18 undergoes beta-plus decay. the child isotope has an atomic mass of done intro donne
Answers: 1

Chemistry, 23.06.2019 15:00
How much more basic is a solution with ph 8 than a solution with ph 7
Answers: 1
You know the right answer?
The chemical equation below shows the burning of magnesium (mg) with oxygen (o2) to form magnesium o...
Questions


Mathematics, 29.01.2021 05:10


Mathematics, 29.01.2021 05:10

Mathematics, 29.01.2021 05:10


Social Studies, 29.01.2021 05:10


Mathematics, 29.01.2021 05:10




Mathematics, 29.01.2021 05:10

History, 29.01.2021 05:10


Mathematics, 29.01.2021 05:10

Social Studies, 29.01.2021 05:10

Mathematics, 29.01.2021 05:10


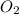 will be required to react completely with 4 moles of Mg.
will be required to react completely with 4 moles of Mg.