
Determine the concentrations of k2so4, k , and so42– in a solution prepared by dissolving 1.80 × 10–4 g k2so4 in 1.50 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm). note: determine the formal concentration of so42–. ignore any reactions with water.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 23.06.2019 06:00
What physical property of gold makes panning a useful way to get gold from streams?
Answers: 2

Chemistry, 23.06.2019 11:00
Asolubility table shows that almost all compounds of group 1 metals are soluble in water. this general rule tells you that mgi2 is soluble rbno3 is soluble cacl2 is soluble co2 is soluble
Answers: 1
You know the right answer?
Determine the concentrations of k2so4, k , and so42– in a solution prepared by dissolving 1.80 × 10–...
Questions

Social Studies, 08.10.2021 19:10

Biology, 08.10.2021 19:10




Biology, 08.10.2021 19:10


Geography, 08.10.2021 19:10


History, 08.10.2021 19:10

Mathematics, 08.10.2021 19:10



Mathematics, 08.10.2021 19:10



Mathematics, 08.10.2021 19:10

English, 08.10.2021 19:10

English, 08.10.2021 19:10

Mathematics, 08.10.2021 19:10

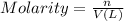
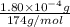
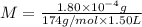
![[K_2SO_4]=M=6.8965\times 10^{-7} mol/L](/tpl/images/0431/3131/1bbe3.png)
![[K^+]=2\times M=2\times 6.8965\times 10^{-7} mol/L=1.3793\times 10^{-6} mol/L](/tpl/images/0431/3131/f96a0.png)
![[SO_4^{2-}]=1\times 6.8965\times 10^{-7} mol/L=6.8965\times 10^{-7} mol/L](/tpl/images/0431/3131/472ff.png)
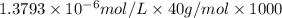
![[K^+]=0.05517 ppm](/tpl/images/0431/3131/6868b.png)
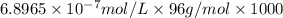
![[SO_4^{2-}]=0.06620 ppm](/tpl/images/0431/3131/956d0.png)


