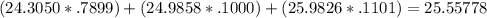Chemistry, 02.02.2020 22:56 RockieLuv7292
Magnesium has three naturally occurring isotopes: mg-24 with mass 24.3050 amu and a natural abundance of 78.99 %, mg-25 with mass 24.9858 amu and a natural abundance of 10.00 %, and mg-26 with mass 25.9826 amu and a natural abundance of 11.01 %.
calculate the atomic mass of magnesium.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 08:40
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2
You know the right answer?
Magnesium has three naturally occurring isotopes: mg-24 with mass 24.3050 amu and a natural abundan...
Questions

Mathematics, 02.08.2019 17:30


Arts, 02.08.2019 17:30

Advanced Placement (AP), 02.08.2019 17:30



Mathematics, 02.08.2019 17:30


History, 02.08.2019 17:30

Mathematics, 02.08.2019 17:30

History, 02.08.2019 17:30

Arts, 02.08.2019 17:30

Mathematics, 02.08.2019 17:30


Mathematics, 02.08.2019 17:30





History, 02.08.2019 17:30