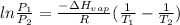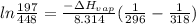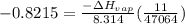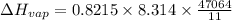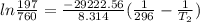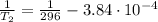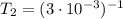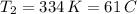Chemistry, 27.08.2019 03:30 lindseyr190
Chloroform, chcl3, was once used as an anesthetic. in spy movies it is the liquid put in handkerchiefs to render victims unconscious. its vapor pressure is 197 mmhg at 23 degrees c and 448 mmhg at 45 degrees
c. estimate its
a. heat of vaporization
b. normal boiling point i calculated the heat of vaporization to be 29.3 kj/mol. i'm having some trouble figuring out the normal boiling point, however. i know that the normal boiling point is when, at 1 atm, a liquid boils at a temperature at which its vapor pressure is equal to the pressure above its surface. so p1=p2=1 atm, if p1= vapor pressure and p2= atmospheric pressure/pressure above surface. i figured i could plug this into pv=nrt and solve, but i'm not given a lot of information. i considered assigning arbitrary values for n and v, so i would have
t= (1.00 atm)(1.00 l)/(1.00 ) but is that really the best way to do this problem, or would it even work at all?
i would think you could use the clausius-clapeyron equation, just as you did for delta h vap, but this time one of the ps will be 760 mm and calculate t for that p.
you, that makes sense, but how do i account for p2 and t2 in the equation if i don't know those values either?
but you have two vapor pressures at two temperatures. i would pick 23 c (change to kelvin, of course) and 197 mm for t1 and p1. then 760 mm and t2 for the others. you have all of the other numbers. check my thinking.
oh, of course, i had completely forgotten about that. you.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2
You know the right answer?
Chloroform, chcl3, was once used as an anesthetic. in spy movies it is the liquid put in handkerchie...
Questions

History, 04.09.2019 17:30

Biology, 04.09.2019 17:30

Mathematics, 04.09.2019 17:30

Geography, 04.09.2019 17:30








History, 04.09.2019 17:30

Spanish, 04.09.2019 17:30




Geography, 04.09.2019 17:30

Spanish, 04.09.2019 17:30


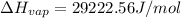
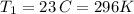
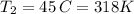
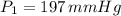
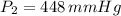
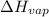 : heat of vaporization
: heat of vaporization