
Chemistry, 05.10.2019 06:30 juniorcehand04
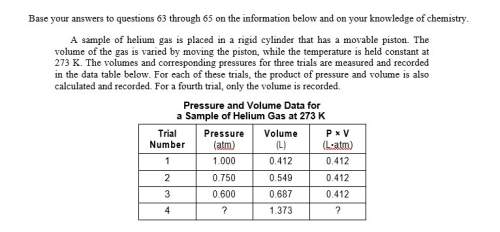
Asample of helium gas is placed in a rigid cylinder that has a movable piston. the volume of the gas is varied by moving the piston, while the temperature is held constant at 273 k. the volumes and corresponding pressures for three trials are measured and recorded in the data table below. for each of these trials, the product of pressure and volume is also calculated and recorded. for a fourth trial, only the volume is recorded.
state evidence found in the data table that allows the product of pressure and volume for the fourth trial to be predicted.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1


Chemistry, 22.06.2019 13:30
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Asample of helium gas is placed in a rigid cylinder that has a movable piston. the volume of the gas...
Questions

Spanish, 28.03.2020 00:17

Health, 28.03.2020 00:17

Mathematics, 28.03.2020 00:17

Computers and Technology, 28.03.2020 00:17

Mathematics, 28.03.2020 00:17


Mathematics, 28.03.2020 00:17





Mathematics, 28.03.2020 00:18

Mathematics, 28.03.2020 00:18

Mathematics, 28.03.2020 00:18


Computers and Technology, 28.03.2020 00:18


Mathematics, 28.03.2020 00:18

Mathematics, 28.03.2020 00:18



