
Chemistry, 27.09.2019 04:50 tayveon122
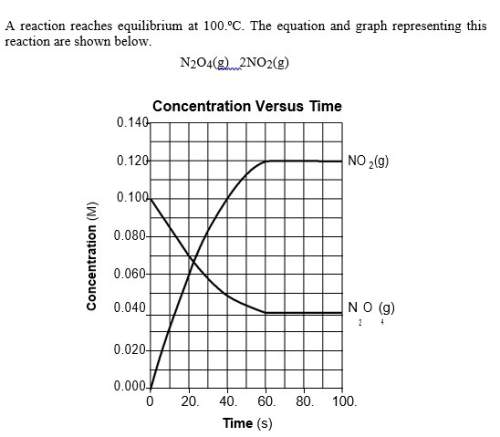
The graph shows that the reaction is at equilibrium after 60. seconds because the concentrations of both no2(g) and n2o4(g) are
(1) increasing
(2) decreasing
(3) constant
(4) zero

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2
You know the right answer?
The graph shows that the reaction is at equilibrium after 60. seconds because the concentrations of...
Questions

Mathematics, 22.06.2021 19:40




Mathematics, 22.06.2021 19:40

Mathematics, 22.06.2021 19:40




Chemistry, 22.06.2021 19:40

English, 22.06.2021 19:40

Geography, 22.06.2021 19:40

Mathematics, 22.06.2021 19:40


Mathematics, 22.06.2021 19:40


Mathematics, 22.06.2021 19:40

Mathematics, 22.06.2021 19:40

Computers and Technology, 22.06.2021 19:40

Health, 22.06.2021 19:40



