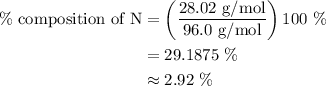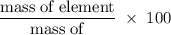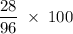Chemistry, 15.10.2019 02:30 lexhorton2002
What is the percent composition by mass of nitrogen in (nh4)2co3 (gram-formula mass = 96.0 g/mol)?
(1) 14.6%
(2) 29.2%
(3) 58.4%
(4) 87.5%

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

You know the right answer?
What is the percent composition by mass of nitrogen in (nh4)2co3 (gram-formula mass = 96.0 g/mol)?...
Questions



Mathematics, 26.06.2019 21:00


Mathematics, 26.06.2019 21:00


Geography, 26.06.2019 21:00



Mathematics, 26.06.2019 21:00

Mathematics, 26.06.2019 21:00

Mathematics, 26.06.2019 21:00

Chemistry, 26.06.2019 21:00






Mathematics, 26.06.2019 21:00

 is
is 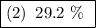 .
.
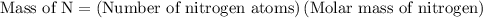 …… (1)
…… (1)
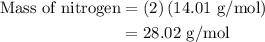
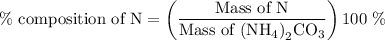 …… (2)
…… (2)