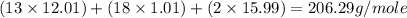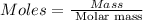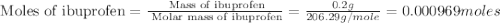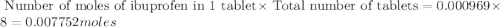1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers. each tablet contains 200 mg of ibuprofen, and a typical adult dose is two tablets every six hours.
• determine the molar mass of ibuprofen. show all steps to find the answer.
• calculate the number of moles of ibuprofen in a single tablet. show all steps to find the answer.
• calculate the number of moles of ibuprofen that an adult would have taken if she took four doses of ibuprofen in one day. show all steps to find the answer.
asap need to get this done fast

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
Which of the following pressures is equal to 760 mm hg? 2.0 atm 101.3 pa 101,300 kpa 101,300 pa
Answers: 2

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 07:00
What is the main purpose of patent attorneys? defend the company against legal claims manage financial investments invent new products protect rights to new products and processes
Answers: 1

You know the right answer?
1. ibuprofen (c13h18o2) is the active ingredient in many nonprescription pain relievers. each tablet...
Questions

Mathematics, 10.12.2021 06:30

Mathematics, 10.12.2021 06:30

Mathematics, 10.12.2021 06:30

Mathematics, 10.12.2021 06:30


Biology, 10.12.2021 06:30

Mathematics, 10.12.2021 06:30

Mathematics, 10.12.2021 06:30

History, 10.12.2021 06:30

Mathematics, 10.12.2021 06:30

Mathematics, 10.12.2021 06:30








Mathematics, 10.12.2021 06:30

History, 10.12.2021 06:30

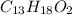 =
=