
Chemistry, 07.10.2019 08:02 ilovejustinbieber42
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the solubility product constant of cu3(aso4)2.
9.1 x 10^-4 m
3.4 x 10^-2 m
3.7 x 10^-8 m
8.7 x 10^-2 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

You know the right answer?
Calculate the molar solubility of copper(ii) arsenate (cu3(aso4)2) in water. use 7.6 x 10^-36 as the...
Questions


Mathematics, 19.06.2020 21:57

Mathematics, 19.06.2020 21:57


History, 19.06.2020 21:57





Chemistry, 19.06.2020 21:57


Mathematics, 19.06.2020 21:57


Mathematics, 19.06.2020 21:57



Mathematics, 19.06.2020 21:57



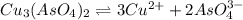
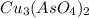 will be given by:
will be given by: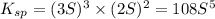
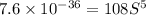
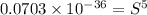
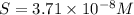
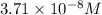 .
.


