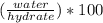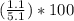Chemistry, 05.02.2020 13:01 andregijoe41
Asample of a hydrated compound has a mass of 5.1 g. during heating, it loses 1.1 g, leaving 4.0 g. what is the percentage by mass of water in the original hydrate?
6.11%
21.6%
27.5%
78.4%

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1


Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2
You know the right answer?
Asample of a hydrated compound has a mass of 5.1 g. during heating, it loses 1.1 g, leaving 4.0 g. w...
Questions


Computers and Technology, 27.07.2019 22:00


Geography, 27.07.2019 22:00







History, 27.07.2019 22:00

Biology, 27.07.2019 22:00


Chemistry, 27.07.2019 22:00

Health, 27.07.2019 22:00

Mathematics, 27.07.2019 22:00



English, 27.07.2019 22:00

Mathematics, 27.07.2019 22:00