
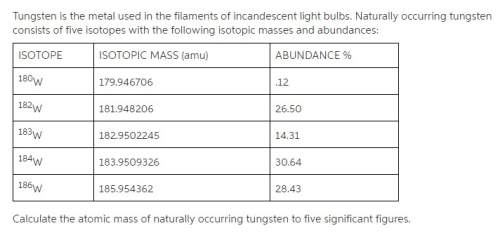
Tungsten is the metal used in the filaments of incandescent light bulbs. naturally occurring tungsten consists of five isotopes with the following isotopic masses and abundances:
calculate the atomic mass of naturally occurring tungsten to five significant figures.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Chemistry, 22.06.2019 06:30
Summarize possible ways in which phases of matter could combine to form a solution.
Answers: 2

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
Tungsten is the metal used in the filaments of incandescent light bulbs. naturally occurring tungste...
Questions


English, 27.07.2020 19:01

History, 27.07.2020 19:01




Mathematics, 27.07.2020 19:01

History, 27.07.2020 19:01


History, 27.07.2020 19:01

History, 27.07.2020 19:01


Social Studies, 27.07.2020 19:01

Mathematics, 27.07.2020 19:01



Mathematics, 27.07.2020 19:01


World Languages, 27.07.2020 19:01



