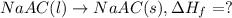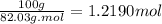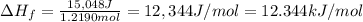Chemistry, 18.10.2019 12:00 christi1175
Acalorimeter contains 500 g of water at 25°c. you place a hand warmer containing 100 g of liquid sodium acetate (naac) inside the calorimeter. when the sodium acetate finishes crystallizing, the temperature of the water inside the calorimeter is 32.2°c. the specific heat of water is
4.18 j/g-°c. what is the enthalpy of fusion (δhf) of the sodium acetate? show your work.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Particles vibrate in a rigid structure and do not move relative to their neighbors.
Answers: 1

Chemistry, 22.06.2019 19:30
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1
You know the right answer?
Acalorimeter contains 500 g of water at 25°c. you place a hand warmer containing 100 g of liquid sod...
Questions







Business, 03.07.2019 21:00

Health, 03.07.2019 21:00


Social Studies, 03.07.2019 21:00

Social Studies, 03.07.2019 21:00





History, 03.07.2019 21:00


Business, 03.07.2019 21:00

Mathematics, 03.07.2019 21:00

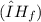 of the sodium acetate is 12.344 kJ/mol.
of the sodium acetate is 12.344 kJ/mol.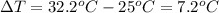
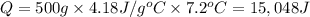
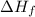 is the heat energy required to melt the one mole of substance.
is the heat energy required to melt the one mole of substance.