
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2o → oh- + sh- which of the following statements is true? na2s is a base because it ionizes to release oh-. na2s is an acid because it is a proton donor. na2s is a base because it increases the hydroxide concentration. none of these

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Idon't really understand this can you me and show your work.☺☺[ chemistry b] subject [ electron transfer in lonic bonds]grade( 12)
Answers: 1

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2
You know the right answer?
D: when sodium sulfide dissociates in water, the sulfur ion reacts with water as follows: s-2 + h2...
Questions

Mathematics, 14.09.2019 21:20








Computers and Technology, 14.09.2019 22:10





Social Studies, 14.09.2019 22:10



Mathematics, 14.09.2019 22:10

Geography, 14.09.2019 22:10


History, 14.09.2019 22:10

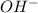 ) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution.
) upon dissociation in water. This means that a base increases the hydroxide ion concentration in a solution. 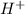 ) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.
) upon dissociation in water. This means that an acid increases the hydrogen ion concentration in a solution.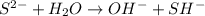
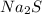 ) dissociates to give hydroxide ions.
) dissociates to give hydroxide ions. 

