
Which statement is correct regarding the reaction below? 3a + 2b yields c + 2d the rate of formation of d is twice the rate of disappearance of a. the rate of disappearance of a is one-third the rate of formation of c. the rate of formation of c is twice the rate of appearance of d. the rate of disappearance of b is twice the rate of appearance of c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Covalent network solids typically have melting points and boiling points. the chemical formula of a network solid indicates in the molecule.
Answers: 3

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 21:00
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

You know the right answer?
Which statement is correct regarding the reaction below? 3a + 2b yields c + 2d the rate of formatio...
Questions

Social Studies, 11.03.2021 17:00


Mathematics, 11.03.2021 17:00

Mathematics, 11.03.2021 17:00

Mathematics, 11.03.2021 17:00


English, 11.03.2021 17:00


Mathematics, 11.03.2021 17:00






History, 11.03.2021 17:00

Mathematics, 11.03.2021 17:00



Mathematics, 11.03.2021 17:00

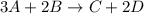
![-\frac{1d[A]}{3dt}](/tpl/images/0398/5946/75d52.png)
![-\frac{1d[B]}{2dt}](/tpl/images/0398/5946/7d814.png)
![\frac{1d[C]}{dt}](/tpl/images/0398/5946/9cd2e.png)
![\frac{1d[D]}{2dt}](/tpl/images/0398/5946/1cfac.png)
![\frac{1d[D]}{dt}=-\frac{2d[A]}{3dt}](/tpl/images/0398/5946/7ef23.png)
![-\frac{1d[A]}{dt}=\frac{3d[C]}{dt}](/tpl/images/0398/5946/87648.png)
![\frac{1d[C]}{dt}=\frac{1d[D]}{2dt}](/tpl/images/0398/5946/1a7b9.png)
![-\frac{1d[B]}{dt}=\frac{2d[C]}{dt}](/tpl/images/0398/5946/56579.png)


