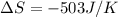Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
What is the value for ∆soreaction for the following reaction, given the standard entropy values? 2...
Questions




Mathematics, 07.11.2020 03:20





Mathematics, 07.11.2020 03:20



English, 07.11.2020 03:20

Social Studies, 07.11.2020 03:20

Mathematics, 07.11.2020 03:20


Mathematics, 07.11.2020 03:20



Mathematics, 07.11.2020 03:20

History, 07.11.2020 03:20

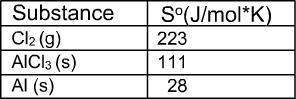
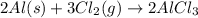
![\Delta S=\sum [n\times S^0(product)]-\sum [n\times \Delta S^0(reactant)]](/tpl/images/0286/6168/a3291.png)
![\Delta S=[(n_{AlCl_3}\times S_{AlCl_3})]-[(n_{Cl_2}\times S_{Cl_2})+[(n_{Al}\times S_{Al})]](/tpl/images/0286/6168/3ce6d.png)
![\Delta S=[(2\times 111)]-[(3\times 223)+(2\times 28)]](/tpl/images/0286/6168/a86a0.png)