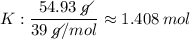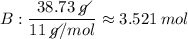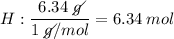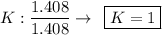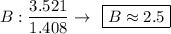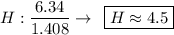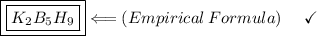Chemistry, 28.09.2019 00:00 romeojose2005
Find the empirical formula of a compound which contains 54.93% potassium, 38.73% boron and 6.34% hydrogen.
a.
kbh
b.
kb2h4
c.
kb3h9
d.
k2b5h9

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 23.06.2019 00:30
How many moles of co2 are produced during the complete combustion of 3.6 moles of c2h6
Answers: 1

You know the right answer?
Find the empirical formula of a compound which contains 54.93% potassium, 38.73% boron and 6.34% hyd...
Questions



Mathematics, 09.10.2019 02:30


Mathematics, 09.10.2019 02:30

Mathematics, 09.10.2019 02:30

History, 09.10.2019 02:30


Biology, 09.10.2019 02:30

Spanish, 09.10.2019 02:30

Mathematics, 09.10.2019 02:30

Mathematics, 09.10.2019 02:30

Spanish, 09.10.2019 02:30




Mathematics, 09.10.2019 02:30