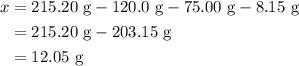Chemistry, 10.12.2019 23:31 sidallen05
When a solution of barium nitrate and a solution of copper (ii) sulfate are mixed, a chemical reaction produces solid barium sulfate, which sinks to the bottom, and a solution of copper (ii) nitrate. suppose some barium nitrate is dissolved in 120.00 g of water and 8.15 g of copper (ii) sulfate is dissolved in 75.00 g of water. the solutions are poured together, and a white solid forms. after the solid is filtered off, it is found to have a mass of 10.76 g. the mass of the solution that passed through the filter is 204.44 g. what mass of barium nitrate was used in the reaction?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 09:30
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1


Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
When a solution of barium nitrate and a solution of copper (ii) sulfate are mixed, a chemical reacti...
Questions

Health, 03.02.2021 16:00

Business, 03.02.2021 16:00



Chemistry, 03.02.2021 16:00

Mathematics, 03.02.2021 16:00


Mathematics, 03.02.2021 16:00

English, 03.02.2021 16:00

English, 03.02.2021 16:00



Mathematics, 03.02.2021 16:00

Mathematics, 03.02.2021 16:00

Mathematics, 03.02.2021 16:00




Mathematics, 03.02.2021 16:00

Mathematics, 03.02.2021 16:00

 .
.
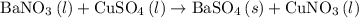
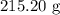 , and the mass of solid
, and the mass of solid 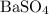 precipitate separated was
precipitate separated was 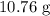 . Hence total mass of products is the sum of these two reactants.
. Hence total mass of products is the sum of these two reactants.
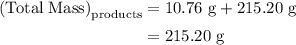
 . Mass of reactants involves mass of barium nitrate, water used for dissolving barium nitrate, mass of copper sulfate and water used for dissolving copper sulfate. The total mass of reactants is calculated as follows:
. Mass of reactants involves mass of barium nitrate, water used for dissolving barium nitrate, mass of copper sulfate and water used for dissolving copper sulfate. The total mass of reactants is calculated as follows:
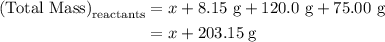
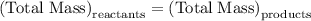 …… (1)
…… (1)
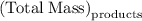 and
and 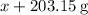 for
for 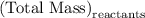 in equation
in equation 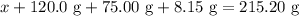
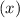 .
.