
Chemistry, 05.02.2020 02:01 zakarycrane9576
Which of the following solutes will lower the freezing point of water the most?
a) the molecular compound sucrose (c₁₂h₂₂22o₁₁)
b) the iconic compound magnesium sulfate (mgso₄4)
c)the iconic lithium chloride (lici)
d)the iconic compound calcium fluoride(caf₂2)

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Frictional forces acting on an object are often converted into energy, which causes the temperature of the object to rise slightly.
Answers: 2

Chemistry, 22.06.2019 09:10
Select the correct answer from each drop-down menu.describe what happens to a carbon-11 atom when it undergoes positron emission.the decay of a carbon-11 atom _1_, and this causes it to emit _2_.options for 1: > changes a neutron into a proton> changes a proton into a neutron> is hit with a neutron> reconfigures its protons and neutronsoptions for 2: > a negatively charged electron-sized particle> a positively charged election-sized particle> two atoms and several neutrons> two neutrons and two protons
Answers: 3

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2
You know the right answer?
Which of the following solutes will lower the freezing point of water the most?
a) the...
a) the...
Questions




Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00

Chemistry, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00

English, 15.04.2021 01:00

Health, 15.04.2021 01:00



Mathematics, 15.04.2021 01:00

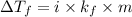
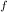 = change in freezing point
= change in freezing point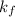 = freezing point constant
= freezing point constant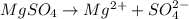 Thus i= 2
Thus i= 2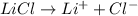 , thus i=2.
, thus i=2.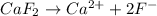 , thus i=3.
, thus i=3.

