
Chemistry, 17.10.2019 04:30 Jacobolobo7
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthalpy for the decomposition of 1 mole of mgo(s) into mg(s) and o2(g)?
consider this combination reaction:
what is the enthalpy for the decomposition of 1 mole of into and ?
-1204 kj/mol
602 kj/mol
1204 kj/mol
-602 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:10
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 23.06.2019 03:00
In november 1987, a massive iceberg broke loose from the antartic ice mass and floated free in the ocean. the chunk of ice was estimated to be 98 mi long, 25 mi wide, and 750 ft thick. a typical backyard swimming pool contains about 24,000 gallons of water. how many of these pools could you fill from the water in this iceberg? (assume the iceberg is a rectangular solid of the above dimensions and consists of water only). express answer in scientific notation.
Answers: 3
You know the right answer?
Consider this combination reaction:
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
2mg(s)+o2(g)→2mgo(s) δh=−1204 kj
what is the enthal...
Questions




Biology, 17.11.2020 23:20

Mathematics, 17.11.2020 23:20


Mathematics, 17.11.2020 23:20


Advanced Placement (AP), 17.11.2020 23:20



Mathematics, 17.11.2020 23:20

Computers and Technology, 17.11.2020 23:20

Mathematics, 17.11.2020 23:20


Chemistry, 17.11.2020 23:20

Biology, 17.11.2020 23:20



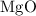 is
is 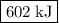 .
. of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.
of an overall reaction is obtained by adding the enthalpy change for each individual step reaction involved to obtain the overall reaction.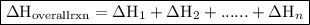
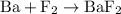
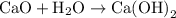
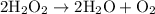
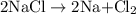
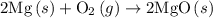
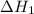 is
is 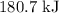 .
.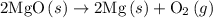
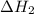 .
.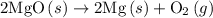 ......(2)
......(2)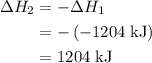
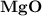 dissociates to give two moles of
dissociates to give two moles of 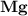 and one mole of
and one mole of 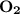 and therefore the enthalpy for the decomposition of one mole of is as follows:
and therefore the enthalpy for the decomposition of one mole of is as follows: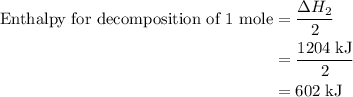
 .
.

