
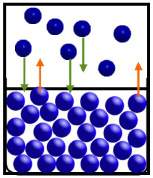
In the diagram below, particles of the substance are moving from the liquid phase to the gas phase at the same rate as they move from the gas phase to the liquid phase.
mc003-1.jpg
the gas and liquid are
~ equilibrium.
~ a high vapor pressure.
~ a low vapor pressure.
~ zero vapor pressure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2


Chemistry, 22.06.2019 09:00
At 300 mm hg, a gas has a volume of 380 l, what is the volume at standard pressure
Answers: 1

Chemistry, 22.06.2019 10:30
Which describes fat? a: a carbohydrate that produces energy b: a nucleic acid that directs cell function c: a lipid that stores energy d: a protein that speeds up a chemical reaction
Answers: 1
You know the right answer?
In the diagram below, particles of the substance are moving from the liquid phase to the gas phase a...
Questions

Mathematics, 16.12.2020 22:30

Mathematics, 16.12.2020 22:30

Mathematics, 16.12.2020 22:30

Social Studies, 16.12.2020 22:30


Mathematics, 16.12.2020 22:30

Mathematics, 16.12.2020 22:40

Chemistry, 16.12.2020 22:40


Mathematics, 16.12.2020 22:40



Chemistry, 16.12.2020 22:40



English, 16.12.2020 22:40






