15. using the information below, calculate δhf° for pbo(s)
pbo(s) + co(g) → pb(s) + co2(...

Chemistry, 09.01.2020 01:31 lindalou6483
15. using the information below, calculate δhf° for pbo(s)
pbo(s) + co(g) → pb(s) + co2(g) δh° = –131.4 kj
δhf° for co2(g) = –393.5 kj/mol
δhf° for co(g) = –110.5 kj/mol
a) –151.6 kj/mol
b) –283.0 kj/mol
c) +283.0 kj/mol
d) –372.6 kj/mol
e) +252.1 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
What are the variables in gay-lussac’s law? pressure and volume pressure, temperature, and volume pressure and temperature volume, temperature, and moles of gas
Answers: 1


Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
Questions


Mathematics, 07.10.2021 23:20


Physics, 07.10.2021 23:20

Physics, 07.10.2021 23:20


Computers and Technology, 07.10.2021 23:20


Business, 07.10.2021 23:20


Mathematics, 07.10.2021 23:20







Mathematics, 07.10.2021 23:20


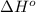
![\Delta H^o_{rxn}=\sum [n\times \Delta H^o_f(product)]-\sum [n\times \Delta H^o_f(reactant)]](/tpl/images/0447/7531/45485.png)
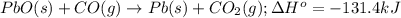
![\Delta H^o_{rxn}=[(1\times \Delta H^o_f_{(Pb(s))})+(1\times \Delta H^o_f_{(CO_2(g))})]-[(1\times \Delta H^o_f_{(PbO(s))})+(1\times \Delta H^o_f_{(CO(g))})]](/tpl/images/0447/7531/c72a3.png)
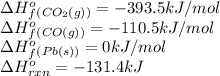
![-131.4=[(1\times \Delta H^o_f_{(0)})+(1\times (-393.5))]-[(1\times \Delta H^o_f_{(PbO(s))})+(1\times (-110.5))]\\\\\Delta H^o_f_{(PbO(s))}=-151.6kJ/mol](/tpl/images/0447/7531/d8c0d.png)


