
Chemistry, 19.04.2021 21:20 skrillex88
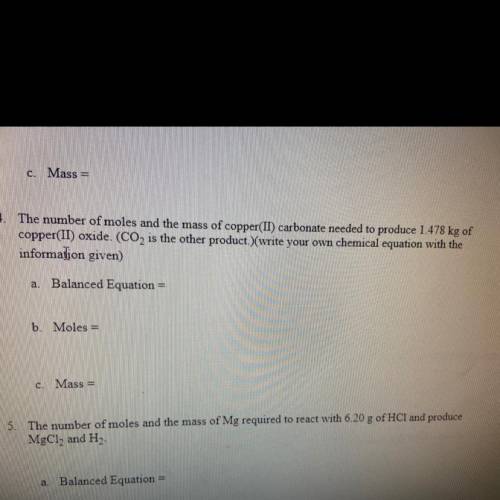
The number of moles and mass of copper carbonate needed to produce 1.478 KG of copper oxide C O two is the other products are your own chemical equation with the information given

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2
You know the right answer?
The number of moles and mass of copper carbonate needed to produce 1.478 KG of copper oxide C O two...
Questions



Mathematics, 21.08.2020 20:01

Mathematics, 21.08.2020 20:01








Social Studies, 21.08.2020 20:01




Mathematics, 21.08.2020 20:01


Mathematics, 21.08.2020 20:01

Mathematics, 21.08.2020 20:01

Chemistry, 21.08.2020 20:01



