
Chemistry, 19.04.2021 18:30 yakshp3702
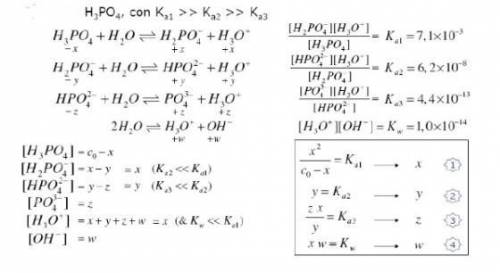
Knowing that the initial concentration of phosphoric acid within a pool is 0.3mg. L-¹, and considering the equilibrium constants and the equilibrium expressions placed in the figure, determine the concentration of all specific species in the solution.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:00
In a sample of oxygen gas at room temperature, the average kinetic energy of all the balls stays constant. which postulate of kinetic molecular theory best explains how this is possible? a. attractive forces between gas particles are negligible because the particles of an ideal gas are moving so quickly. b. collisions between gas particles are elastic; there is no net gain or loss of kinetic energy. c. gases consist of a large number of small particles, with a lot of space between the particles. d. gas particles are in constant, random motion, and higher kinetic energy means faster movement.
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 06:20
Why is it that 85.48 rounded to two significant figures is 85 and not 86?
Answers: 1
You know the right answer?
Knowing that the initial concentration of phosphoric acid
within a pool is 0.3mg. L-¹, and consider...
Questions







Mathematics, 11.05.2021 06:10

Mathematics, 11.05.2021 06:10


Mathematics, 11.05.2021 06:10

Mathematics, 11.05.2021 06:10



Mathematics, 11.05.2021 06:10



Mathematics, 11.05.2021 06:10


Advanced Placement (AP), 11.05.2021 06:10



