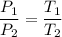Chemistry, 19.04.2021 18:30 mpete1234567890
An ideal gas is held in a rigid container at temperature of 201∘C. Upon cooling the gas, the pressure decreases by a factor of 2.0, while the volume and the amount of gas remain constant. What must be the final temperature of the gas?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1

Chemistry, 23.06.2019 10:40
Question 17 hydrogen is manufactured on an industrial scale by this sequence of reactions: +ch4gh2og ⇌ +cog3h2g k1 +cogh2og ⇌ +co2gh2g k2 the net reaction is: +ch4g2h2og ⇌ +co2g4h2g k write an equation that gives the overall equilibrium constant k in terms of the equilibrium constants k1 and k2. if you need to include any physical constants, be sure you use their standard symbols, which you'll find in the aleks calculator.
Answers: 2

You know the right answer?
An ideal gas is held in a rigid container at temperature of 201∘C. Upon cooling the gas, the pressur...
Questions

Chemistry, 04.03.2020 07:49


Mathematics, 04.03.2020 07:49

Advanced Placement (AP), 04.03.2020 07:49

Mathematics, 04.03.2020 07:49


Mathematics, 04.03.2020 07:49

Business, 04.03.2020 07:50



Mathematics, 04.03.2020 07:50

History, 04.03.2020 07:50



English, 04.03.2020 07:50



English, 04.03.2020 07:50

Chemistry, 04.03.2020 07:51