Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1

Chemistry, 22.06.2019 21:20
The organs inside the body and how they function together
Answers: 3

Chemistry, 23.06.2019 06:40
8. how much enthalpy/heat is transferred when 0.5113gof ammonia (nh3) reacts with excess oxygen according| to the following equation: 4nh3 +502 - 4n0+ 6h20ah = -905.4j
Answers: 1
You know the right answer?
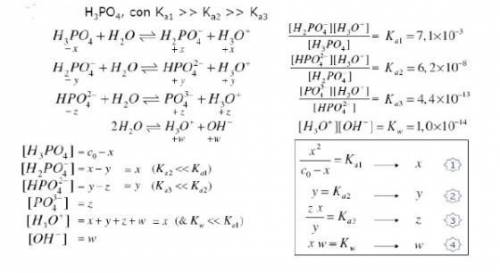
Knowing that the initial concentration of phosphoric acid
within a pool is 0.3mg. L-¹, and consider...
Questions


Chemistry, 24.05.2021 21:40


Mathematics, 24.05.2021 21:40






Mathematics, 24.05.2021 21:40

History, 24.05.2021 21:40

Biology, 24.05.2021 21:40




Computers and Technology, 24.05.2021 21:40



Mathematics, 24.05.2021 21:40