
Chemistry, 19.04.2021 07:10 irelandcrawford5469
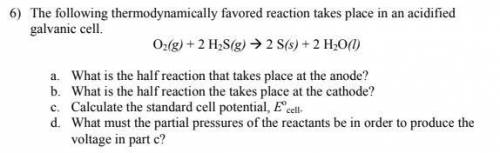
The following thermodynamically favored reaction takes place in an acidified
galvanic cell.
O2(g) + 2 H2S(g) 2 S(s) + 2 H2O(l)
a. What is the half reaction that takes place at the anode?
b. What is the half reaction the takes place at the cathode?
c. Calculate the standard cell potential, Eo
cell.
d. What must the partial pressures of the reactants be in order to produce the
voltage in part c?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
Which of these conditions most likely produces an unstable isotope?
Answers: 2

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 17:30
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
You know the right answer?
The following thermodynamically favored reaction takes place in an acidified
galvanic cell.
Questions



Computers and Technology, 19.02.2020 20:31




History, 19.02.2020 20:31

Social Studies, 19.02.2020 20:31




Mathematics, 19.02.2020 20:32



Computers and Technology, 19.02.2020 20:32

English, 19.02.2020 20:32

Computers and Technology, 19.02.2020 20:32





