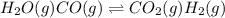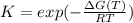Chemistry, 17.04.2021 20:00 andrejr0330jr
For the following exothermic reaction system at equilibrium:
H2O(g) CO(g) CO2(g) H2(g)
Choose the changes that will increase the value of K.
a. Decrease the volume (constant T)
b. Add H2O(g) (constant T)
c. Remove H2(g) (constant T)
d. Add a catalyst (constant T)
e. Add CO2(g) (constant T)
f. Increase the temperature
g. Decrease the temperature

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Long term exposure to waves can cause sunburns and skin cancer. a) visible b) infrared c) gamma rays d) ultraviole
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

You know the right answer?
For the following exothermic reaction system at equilibrium:
H2O(g) CO(g) CO2(g) H2(g)
Choos...
Choos...
Questions


Mathematics, 07.10.2019 14:50







English, 07.10.2019 14:50

Social Studies, 07.10.2019 14:50

Biology, 07.10.2019 14:50

History, 07.10.2019 14:50



Computers and Technology, 07.10.2019 14:50

Business, 07.10.2019 14:50



Mathematics, 07.10.2019 14:50