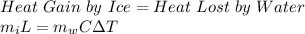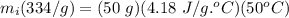Chemistry, 17.04.2021 08:50 eelebron0905
Ice at 0.0 degrees C is combined with 50.0g of water at 75.0 degrees C. Calculate the grams of ice present initially if the entire mixture comes to a final temperature of 25.0 degrees C after the ice melts. Specific heat of water is 4.18 J/goC. SHOW WORK!

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:10
Atank contains 240 liters of fluid in which 10 grams of salt is dissolved. brine containing 1 gram of salt per liter is then pumped into the tank at a rate of 6 l/min; the well-mixed solution is pumped out at the same rate. find the number a(t) of grams of salt in the tank at time t.
Answers: 3

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 17:20
Which of these features are formed when hot groundwater is forced out through cracks in the earth's surface?
Answers: 2
You know the right answer?
Ice at 0.0 degrees C is combined with 50.0g of water at 75.0 degrees C. Calculate the grams of ice p...
Questions

English, 25.12.2020 07:50

Geography, 25.12.2020 07:50


Mathematics, 25.12.2020 07:50


History, 25.12.2020 07:50


Chemistry, 25.12.2020 08:00

Mathematics, 25.12.2020 08:00

Business, 25.12.2020 08:00

Mathematics, 25.12.2020 08:00


Biology, 25.12.2020 08:00

Geography, 25.12.2020 08:00

English, 25.12.2020 08:00


Mathematics, 25.12.2020 08:00