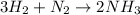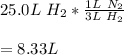Gaseous ammonia, NH3, is obtained from the reaction of gaseous hydrogen and gaseous nitrogen. According to this reaction, what volume, in L, of nitrogen is required to completely react with 25.0 L of hydrogen under STP conditions?
Group of answer choices
8.32 mL
8.32 L
8.20 L
8.00 L

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:30
The famous scientist galileo galilei did several experiments with sloping planes, which he rolled metal balls down so that he could study motion. by changing the slope, he could study how the speed at which the ball rolled was affected. what was the independent variable in galileo's experiment? a. the speed of the ball b. the slope of the plane c. whether the ball moved d. what the ball was made of
Answers: 2

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 2

Chemistry, 23.06.2019 02:00
An alpha particle is: a hydrogen atom a nucleus of helium two neutrons an electron
Answers: 1

Chemistry, 23.06.2019 03:50
What is the equation fort the alkaline zinc/manganese dioxide cell. a) anode b)cathode c)overall equations.
Answers: 2
You know the right answer?
Gaseous ammonia, NH3, is obtained from the reaction of gaseous hydrogen and gaseous nitrogen. Accord...
Questions


Mathematics, 03.03.2021 01:00



Geography, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

History, 03.03.2021 01:00


English, 03.03.2021 01:00


English, 03.03.2021 01:00



Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00

Mathematics, 03.03.2021 01:00



Mathematics, 03.03.2021 01:00

Health, 03.03.2021 01:00