
Chemistry, 16.04.2021 19:30 2021CanadyRaniya
ACTIVITY: SOLUTION CONCENTRATION VS. CONDUCTIVITY
Here is your goal for this lesson:
Graph experimental data and interpret results for peer review
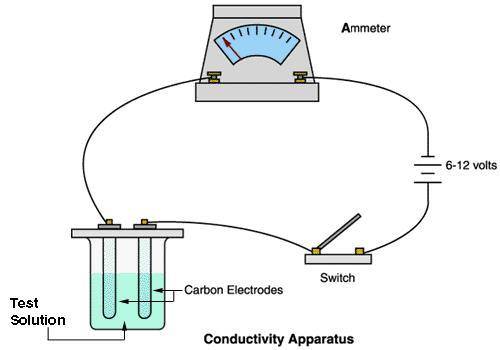
A chemistry student carried out an experiment with a conducting apparatus (ammeter) similar to the one below. This ammeter measures in milliamperes (mA). The following data was taken.
Solution Reading
0.1 M H2SO4 150 mA
0.1 M Ba(OH)2 150 mA
To 30 mL of the Ba(OH)2 solution, 10 mL portions of H2SO4 were added until a total of 50 mL of H2SO4 were used. The following results were recorded.
DATA TABLE
Total H2SO4 Added Meter Reading Observations
0 mL 150 mA Ba(OH)2 and H2SO4 clear, colorless
10 mL 65 mA milky white precipitate forms
20 mL 31 mA more precipitate forms
30 mL 0 mA precipitate heavy and settles
40 mL 29 mA no added precipitate seen to form
50 mL 62 mA no change seen
Questions
1. Did you plot the data?
yes
no
2. Did you label your graph axes?
no
yes
3. Did you give your graph a title?
no
yes
4. Does the Ba(OH)2 solution contain ions?
yes
no
5. Does the H2SO4 solution contain ions?
yes
no
6. Explain the data.
Is there any evidence that a reaction has occurred?
7. Does the conductivity increase or decrease?
8. Does the number of ions in solution increase, decrease, or remain constant?
9. What is the indicator of the number of ions in solution?
10. How does this evidence indicate that the reaction has occurred between ions?
11. The Ba(OH)2 dissociates as Ba+2 + 2 OH-. H2SO4 dissociates as 2 H+ + SO4-2.
Write a balanced equation for this reaction.
12. When the conductivity is at a minimum, what must be true about the amount of Ba(OH)2?
13. Why does it not conduct at this low point?
14. Why does it conduct more before and after this minimum point?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When svante arrhenius first proposed his acid-base theory, he was a doctoral candidate. his professors thought his ideas were unfounded. within a decade, the arrhenius theory of acid-base was widely accepted and praised within the scientific world. arrhenius defined acids as compounds having ionizable hydrogen and bases as compounds with ionizable a) barium. b) hydronium. c) hydroxide. d) oxygen.
Answers: 3

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 21:20
If a simple machine aduces the strength of a force, what must be increased? the speed of the input force the work the simple machine performs the size of the simple machine the distance over which the force is applied
Answers: 1

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1
You know the right answer?
ACTIVITY: SOLUTION CONCENTRATION VS. CONDUCTIVITY
Here is your goal for this lesson:
Gr...
Gr...
Questions

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Spanish, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Chemistry, 18.09.2020 16:01

History, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01

Mathematics, 18.09.2020 16:01



