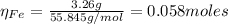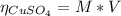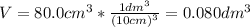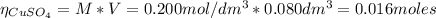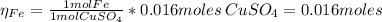Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 13:30
Ants live on acacia trees in south america. the ants feed on sugars secreted by the trees. the trees provide room for the ants to live. the ants sting any other insect or animal that comes to eat the trees. what type of relationship is this?
Answers: 1

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3
You know the right answer?
2.a. 3.26 g of iron powder are added to 80.0 cm3 of 0.200 mol dm-3 copper(II)
sulfate solution. The...
Questions

Social Studies, 05.05.2020 04:26

Mathematics, 05.05.2020 04:26




Mathematics, 05.05.2020 04:26

Mathematics, 05.05.2020 04:26

Mathematics, 05.05.2020 04:26

Mathematics, 05.05.2020 04:26

Mathematics, 05.05.2020 04:26



Mathematics, 05.05.2020 04:26



History, 05.05.2020 04:26




Mathematics, 05.05.2020 04:26

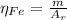
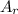 : is the standard atomic weight of iron = 55.845 g/mol
: is the standard atomic weight of iron = 55.845 g/mol