
Chemistry, 16.04.2021 17:50 donaldwilliams31
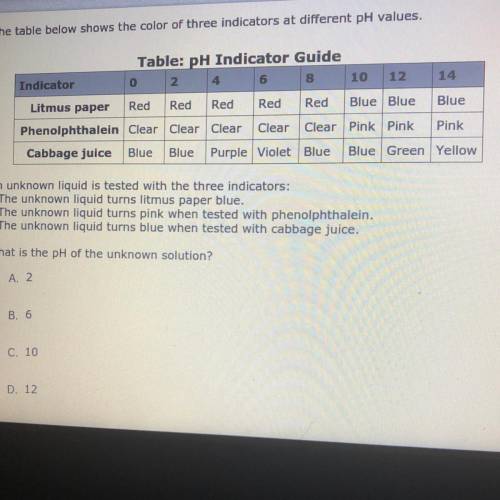
The table below shows the color of three indicators at different pH values.
An unknown liquid is tested with the three indicators:
• The unknown liquid turns litmus paper blue.
• The unknown liquid turns pink when tested with phenolphthalein.
• The unknown liquid turns blue when tested with cabbage juice.
What is the pH of the unknown solution?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
The conventional equilibrium constant expression (kc) for the system below is: 2icl(s) ⇄ i2(s) + cl2(g) [cl2] ([i2] + [cl2])/2[icl] [i2][cl2]/[icl]2 none of the listed answers are correct [i2][cl2]/2[icl]
Answers: 2

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
The table below shows the color of three indicators at different pH values.
An unknown liquid is te...
Questions










Mathematics, 17.03.2020 23:22

Mathematics, 17.03.2020 23:22

Chemistry, 17.03.2020 23:22



Biology, 17.03.2020 23:22

History, 17.03.2020 23:22

Physics, 17.03.2020 23:22


Mathematics, 17.03.2020 23:22



