
Chemistry, 16.04.2021 17:30 SiegeHatake4534
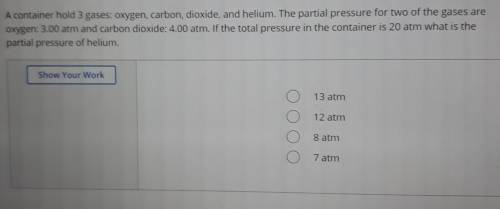
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the gases are oxygen: 3.00 atm and carbon dioxide: 4.00 atm. If the total pressure in the container is 20 atm what is the partial pressure of helium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
Noble gases are the most reactive elements on the periodic table. a. true b. false
Answers: 2

Chemistry, 22.06.2019 06:30
Design techniques and materials that reduce the negative environmental impact of a structure are referred to as
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1
You know the right answer?
A container hold 3 gases: oxygen, carbon, dioxide, and helium. The partial pressure for two of the g...
Questions









English, 24.02.2020 22:27

Mathematics, 24.02.2020 22:27



Mathematics, 24.02.2020 22:27




History, 24.02.2020 22:27


Mathematics, 24.02.2020 22:27

Mathematics, 24.02.2020 22:27



