
Chemistry, 16.04.2021 06:10 endermss1970
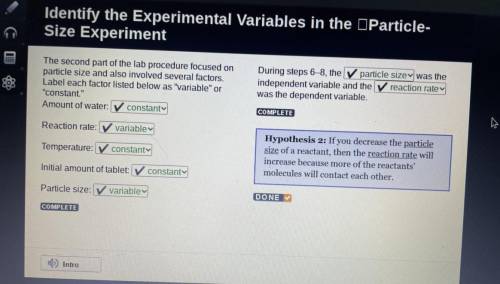
The second part of the lab procedure focused on
particle size and also involved several factors.
Label each factor listed below as "variable" or
"constant."
Amount of water: constant
Reaction rate: variable
Temperature: constanty
Initial amount of tablet: constant
Particle size: variable

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1

Chemistry, 22.06.2019 20:00
In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. how many degrees of freedom are there for such a system? the reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. how many degrees of freedom are there for this system? steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. the following reactions have been suggested as being involved in the chemical transformation:
Answers: 3

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3
You know the right answer?
The second part of the lab procedure focused on
particle size and also involved several factors.
Questions


Chemistry, 03.12.2021 06:40


Mathematics, 03.12.2021 06:40

History, 03.12.2021 06:40

Social Studies, 03.12.2021 06:40

Mathematics, 03.12.2021 06:40

Computers and Technology, 03.12.2021 06:40

Mathematics, 03.12.2021 06:40







Mathematics, 03.12.2021 06:40


Advanced Placement (AP), 03.12.2021 06:40




