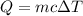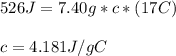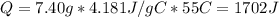Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 21:30
An atomic nucleus is composed ofa)protons.b)protons and neutrons.c)protons and electrons.d)protons, neutrons, and electrons.
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
If it takes 526 j of energy to warm 7.40g of water by 17 degrees celsius, how much energy would be n...
Questions


Biology, 01.10.2021 03:50





Business, 01.10.2021 03:50


Geography, 01.10.2021 03:50

Computers and Technology, 01.10.2021 03:50


Social Studies, 01.10.2021 03:50

Mathematics, 01.10.2021 03:50





Chemistry, 01.10.2021 03:50

History, 01.10.2021 04:00