
Chemistry, 16.04.2021 01:00 karrathomas
Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this: (s)(s)(g) At a certain temperature, a chemist finds that a reaction vessel containing a mixture of calcium carbonate, calcium oxide, and carbon dioxide at equilibrium has the following composition: compound amount Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:50
A2.5% (by mass) solution concentration signifies that there is a 2.5 % (by mass) solution concentration signifies that there is blank of solute in every 100 g of solution. of solute in every 100 g of solution
Answers: 3


Chemistry, 22.06.2019 23:30
If maltose undergoes hydrolysis what subunits does it results to?
Answers: 2
You know the right answer?
Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this: (s)(s)(g) At a cer...
Questions

Mathematics, 31.03.2020 14:57


History, 31.03.2020 14:57

Mathematics, 31.03.2020 14:58



Mathematics, 31.03.2020 14:58


Mathematics, 31.03.2020 14:58

Mathematics, 31.03.2020 14:58

Mathematics, 31.03.2020 14:59



Physics, 31.03.2020 15:00

Geography, 31.03.2020 15:01



German, 31.03.2020 15:03


Mathematics, 31.03.2020 15:05

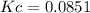
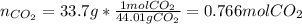
![[CO_2]=\frac{0.766molCO_2}{9.0L}=0.0851M](/tpl/images/1262/6588/3d5ed.png)
![Kc=[CO_2]\\\\Kc=0.0851](/tpl/images/1262/6588/d8c10.png)


