
Chemistry, 15.04.2021 18:20 hdjsjfjruejchhehd
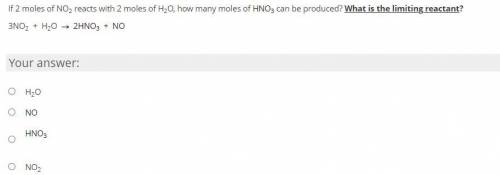
If 2 moles of NO2 reacts with 2 moles of H2O, how many moles of HNO3 can be produced? What is the limiting reactant? -NO LINKS

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
10. according to the law of conservation of mass, how does the mass of the products in a chemical reaction compare to the mass of the reactants?
Answers: 3

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1
You know the right answer?
If 2 moles of NO2 reacts with 2 moles of H2O, how many moles of HNO3 can be produced? What is the li...
Questions



Mathematics, 26.03.2021 22:50

Biology, 26.03.2021 22:50

Mathematics, 26.03.2021 22:50


English, 26.03.2021 22:50

Chemistry, 26.03.2021 22:50

English, 26.03.2021 22:50






Spanish, 26.03.2021 22:50


Mathematics, 26.03.2021 22:50



Mathematics, 26.03.2021 22:50



