When is ksp determined for a system? (select all that apply.)
-any time that the solid and li...

Chemistry, 26.01.2020 23:31 mustafa455486a
When is ksp determined for a system? (select all that apply.)
-any time that the solid and liquid phases are both present
-only after all solid has been dissolved
-when equilibrium is established
-when the two phase system is saturated

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 11:30
The dashed segment of the plotted experiment in the graph in the l
Answers: 3
You know the right answer?
Questions

Mathematics, 05.05.2020 06:36




Mathematics, 05.05.2020 06:36

Chemistry, 05.05.2020 06:36

Mathematics, 05.05.2020 06:36



Computers and Technology, 05.05.2020 06:36

Mathematics, 05.05.2020 06:36




Mathematics, 05.05.2020 06:36



Mathematics, 05.05.2020 06:36

Geography, 05.05.2020 06:37

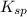 is known as solubility product and it is defined as when a solid and its ions in a solution are in equilibrium with each each other.
is known as solubility product and it is defined as when a solid and its ions in a solution are in equilibrium with each each other. 

